Orphan Drugs
PHARMABIO CONSULTING staff have extensive experience in Orphan Drug Submissions
Services for Orphan Drugs
Designation of Orphan Drug
• Preparation of the detailed Application Form (common document for EU and US)
• Preparation of the document “Application for Designation as Orphan Medicinal Product”
• Pre-submission meeting with the EMA and finalization of documents
• Compilation and Submission of Orphan Drug Designation Dossier to the EMA
Development of Orphan Drug
Developing orphan drugs is always a challenge for different reasons, among which are precisely defining the condition through clinical symptoms & signs, physiology and biochemistry, and hence deciding on the eligibility of the patient for treatment.
Conducting studies of test drug against controls such as placebo may not be ethical, and against a comparator problematic, where there is an existing an unmet medical need and there is no “gold standard” of treatment.
The small numbers of patients raises questions over reaching justifiable conclusions on efficacy and safety.
Marketing Authorisation of Orphan Drug
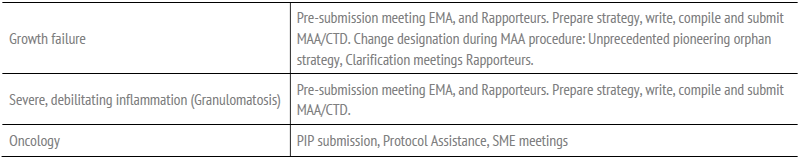
Table Representative Orphan Drug Submissions, starting with application for designation
Development of biosimilars of biological O/D afford new opportunities
Biosimilars are a special class of medicines while O/Ds themselves have restrictions. This introduces new challenges to comparability exercises of the Quality and Clinical program when developing and registering a biosimilars O/D. PharmaBio has expertise in both to offer solutions acceptable to Regulators.

